-
Optical investigation of spin-crossover in cobalt(II) bis-terpy complexes
C. Enachescu, I. Krivokapic, M. Zerara, J.A. Real, N. Amstutz and A. Hauser
Inorganica Chimica Acta, 360 (13) (2007), p3945-3950


DOI:10.1016/j.ica.2007.06.022 | unige:3585 | Abstract | Article HTML | Article PDF
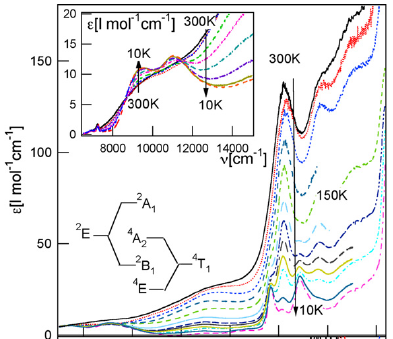
The spin transition of the [Co(terpy)2]2+ complex (terpy = 2,2′:6′,2″-terpyridine) is analysed based on experimental data from optical spectroscopy and magnetic susceptibility measurements. The single crystal absorption spectrum of [Co(terpy)2](ClO4)2 shows an asymmetric absorption band at 14 400 cm−1 with an intensity typical for a spin-allowed d–d transition and a temperature behaviour typical for a thermal spin transition. The single crystal absorption spectra of suggest that in this compound, the complex is essentially in the high-spin state at all temperatures. However, the increase in intensity observed in the region of the low-spin MLCT transition with increasing temperature implies an unusual partial thermal population of the low-spin state of up to about 10% at room temperature. Finally, high-spin → low-spin relaxation curves following pulsed laser excitation for [Co(terpy)2](ClO4)2 dispersed in KBr discs, and as a comparison for the closely related [Co(4-terpyridone)2](ClO4)2 spin-crossover compound are given.